The Implementation of Medical Cannabis and Psychedelics Used as an Adjunct to Standard Therapy in the Treatment of Advanced Metastatic Breast Cancer
By Jamie Brambila at Grace Botanical Pharmaceuticals
INTRODUCTION
A 49-year-old woman was diagnosed and treated for ER +, PR-, HER2 + , BRCA- invasive ductal carcinoma, which progressed metastatically to include bone, liver, and lymph node involvement.
METHODS
Standardized care included a 26-month treatment with targeted chemotherapy and a ketogenic diet. The patient also began a course of cannabinoid-based therapy, consisting initially of a titrated high-dose protocol of mixed cannabidiol (CBD) and d9-tetrahydrocannabinol (THC) chemotypes, as well as psilocybin-assisted psychotherapy at macro and intermittent micro-doses
RESULTS
At the end of the five-month treatment period, PET/CT investigations revealed no evidence of metastatic disease, and chemotherapy was withdrawn. A one-year follow-up CT investigation concluded no evidence of residual or recurrent disease. A recurrence of the disease was noted at 18 months follow-up.
Over these 18 months, the cannabis regimen was titrated down to 60% of the initial protocol. This was subsequently increased to the initial dosing protocol following the detection of recurrent disease, and this titration occurred over a 10-month period where it remained stable. 16 months following the detection of recurrence of the disease, favorable results were observed in the patient with evidence of receding cancer progression.
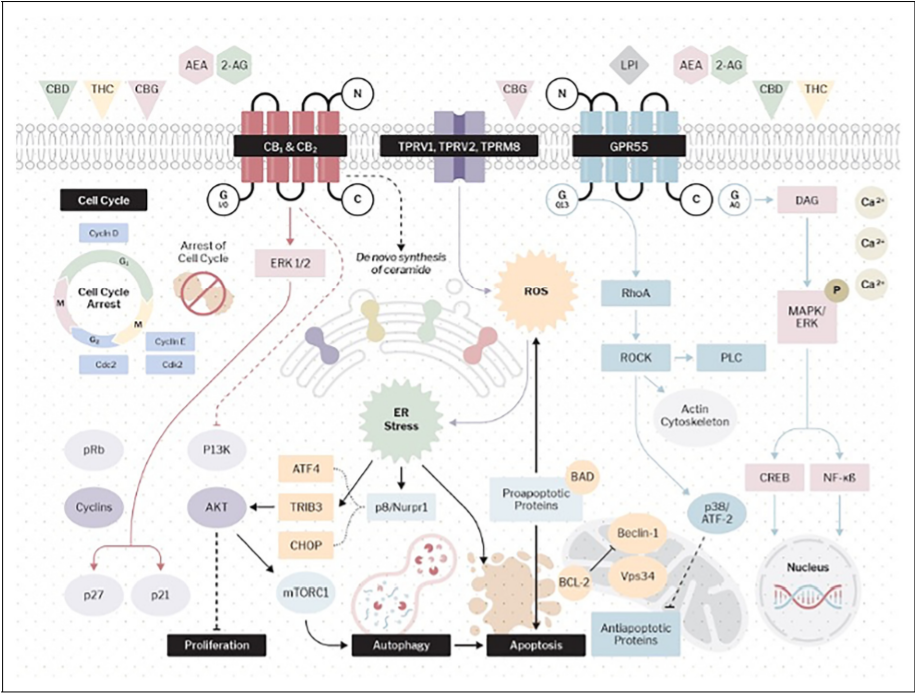
CONCLUSION
Over the last 15 years, there has been a considerable body of in-vitro and in-vivo evidence supporting the anti-neoplastic properties of cannabinoids and, more recently, psychedelics. While this is a sample of N = 1, it provides valuable insights into the real-world prescribing of medical cannabis, indicating appropriate therapeutic doses and a range of adjuvant medications that would be very difficult to reflect in a randomized controlled clinical trial.
